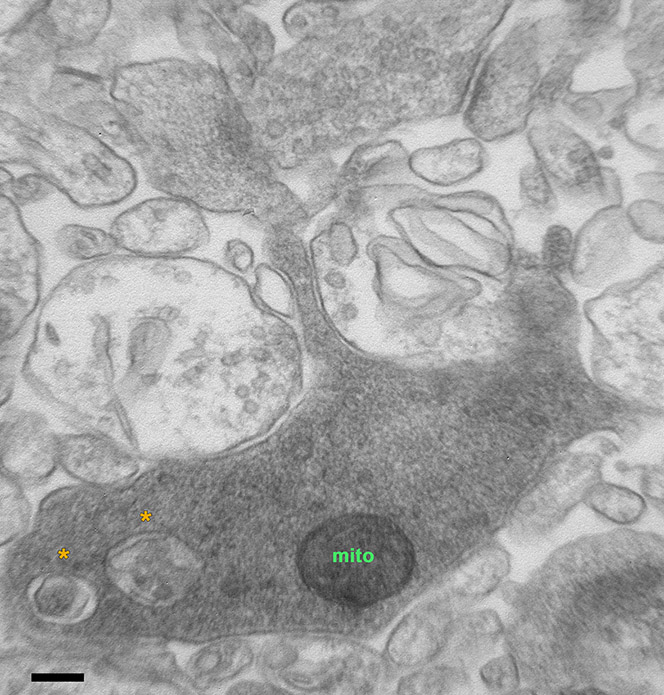
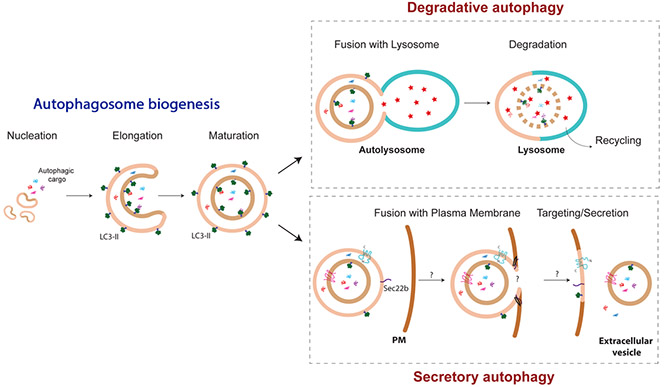
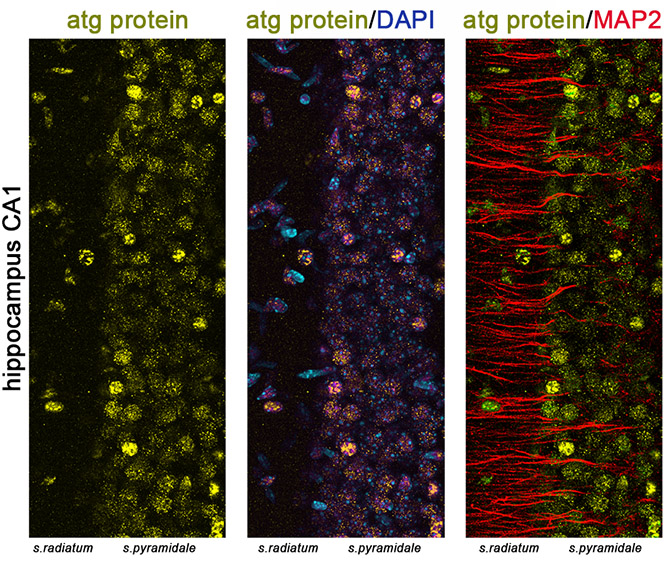
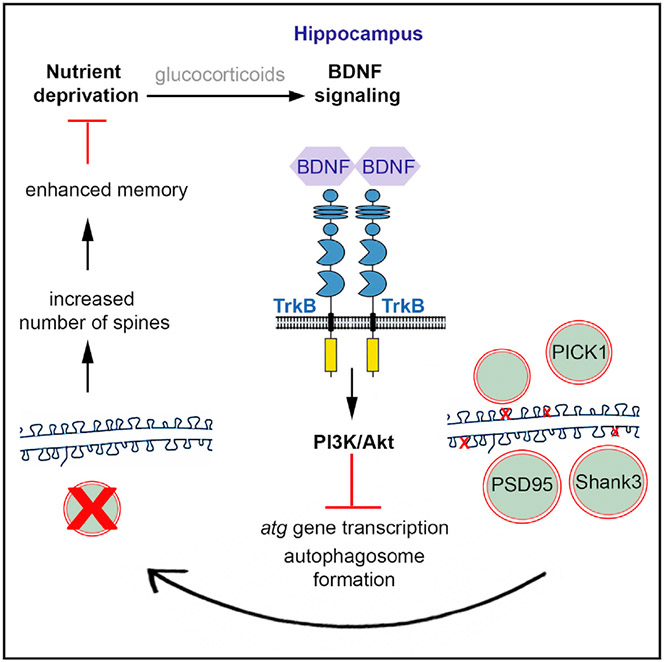
Many forms of long-term synaptic plasticity that underlie key cognitive functions, such as memory formation, memory erasure and behavioral flexibility, require protein turnover, meaning both protein synthesis and degradation. Autophagy is a major pathway for protein degradation, yet its requirement in synaptic plasticity remains unknown. The aim of this project is to characterize the contribution of autophagy in different forms of synaptic plasticity. Similarly, the regulation of autophagosome biogenesis and flux in neurons under conditions of synaptic activation is also elusive and is addressed in our lab. To these ends, we use molecular biology tools, electron microscopy (Figure 1), genetic models, electrophysiology and behavioral analyses.